Session 4 Heat, THERMODYNAMICS and ENTROPY
This session is a natural progression from session 2 in that it takes MECHANICS and inter-twines it with heat. I was lucky to have been taught Thermodynamics by Prof. F.E.Hoare and he religously used his text "A textbook of THERMODYNAMICS", published by E. Arnold, 1952. He was a master of his subject and his text is well worth reading. My passion for the subject started, as I have said, by riding a small motor bike and wondering how engines work.
The web is now a natural starting place for any studies and the series of lectures by Michael Fowler is the best that I have found. Duplicating his notes in this session would seem to be unnecessary as they are freely available --- so, please continue with his 89 pages of an adventure into the subject. I will only supplement a few pictures to reinforce the topic of entropy.
(Just Google " Heat and Thermodynamics by Fowler " and the following .pdf file should be available)
***************************************************************************
Lectures on Heat and Thermodynamics
Physics 152
Michael Fowler, University of Virginia 8/30/08
Contents
HEAT.....................................................................................................................................3
Feeling and seeing temperature changes...................................................................................3
Classic Dramatic Uses of Temperature-Dependent Effects........................................................4
The First Thermometer.............................................................................................................5
Newton’s Anonymous Table of Temperatures..........................................................................7
Fahrenheit’s Excellent Thermometer.........................................................................................7
Amontons’ Air Thermometer: Pressure Increases Linearly with Temperature.............................7
Thermal Equilibrium and the Zeroth Law of Thermodynamics...................................................8
Measuring Heat Flow: a Unit of Heat.......................................................................................8
Specific Heats and Calorimetry............................................................................................. 9
A Connection With Atomic Theory.........................................................................................10
Latent Heat............................................................................................................................11
THERMAL EXPANSION AND THE GAS LAW...............................................................12
Coefficients of Expansion.......................................................................................................12
Gas Pressure Increase with Temperature...............................................................................13 AND MORE!!!!!
**********************************************************************
After reading Michael Fowler's article you should have a fair understanding of Heat and Thermodynamics but I remember when I did the first "walk through the subject of Thermodynamics " I felt mystified that "ENTROPY" had been introduced into the Carnot cycle.... a PV diagram is all that is needed. So a little more eleboration is now given.
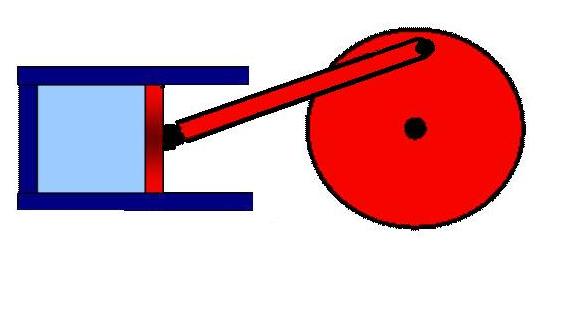
Most courses on the Carnot cycle start with a Carnot engine and this is simply a piston connected to a flywheel as shown.

The notes stress the importance of reversibility and the diagram below suggests how careful one must be to get the piston to move in this manner
If weights are moved to the side shelves in an almost continuous manner and then replaced in the same way then we can hope for a reversible change. If, however, a single large weight is holding the piston down and this is removed it is likely that the piston will spring up and possibly oveshoot it's new stable position only to settle after an oscillating motion. Also, the piston is more likely to experience friction after such motion. Thus, when a weight is added again we may see that the piston attains a lower level and a truely cyclic motion has not been achieved.
Once we are happy that our Carnot engine can trace out a "reversible" path then we can draw the cycle on a PV diagram.
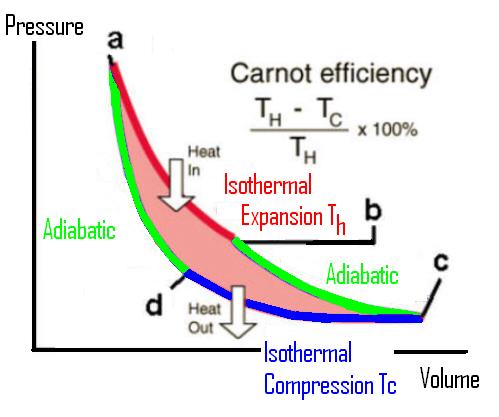
The efficiency is then calculated as the ratio of the work done by the gas compared to the heat given to the gas:-
efficiency = 1 - Tc/Th and this shows that heat can be converted into work but that there is a severe inefficiency with this conversion. Roughly 2/3 of the energy used to produce electricity is wasted and only 1/3 gives an electrical output. This "secret of nature" has very far reaching consequencies as will be discussed at the end of the session.
Thermodynamics would now be expected to continue with an investigation of other cycles -- Otto, Diesel, Stirling, Atkinson .... and show that the Carnot cycle is more efficient then all the rest.
BUT the subject of "entropy" IS ALWAYS introduced at this point and I, for one, wondered "why??? ".
Let us think of Internal Energy first : the internal energy of, say, one's hand can be changed either by holding it infront of the fire or by someone vigorously rubbing it --- Internal energy change depends on (work done ) AND (applied heat) so for the Carnot cycle we have the diagrams
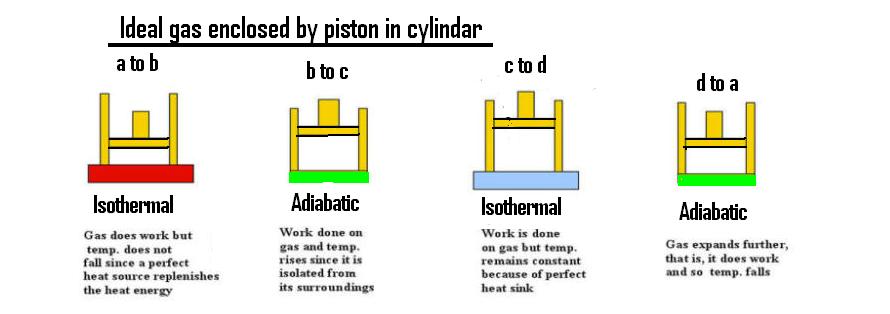
and the resultant change in internal energy
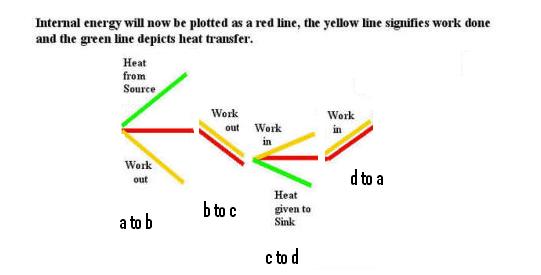
As expected, the internal energy returns to it's inital point after the cycle. If we were supplied with meters to check how the engine was changing with time we would have -- a PRESSURE meter, a meter TO MEASURE VOLUME. a THERMOMETER and an INTERNAL ENERGY meter. On each of these meters we see that the finishing point is exactly the same as the starting point provided we have carefully carried out a reversible cycle. Do we have anything else that we need to moniter? Suprisingly, the quantity Q/T also has this same property of coming back to the starting point since it has been shown that Qh/Th is equal to Qc/Tc during the cycle. In all cases the changes are very similar to mountain climbing as illustrated below.
We can go up the hill by any route to get to a higher level and then return to the lower level and we have achieved our cycle. This type of behavior is common to potential energy in MECHANICS or potential energy in ELECTROSTATICS and apparently so in THERMODYNAMICS. So, the Carnot cycle gives us an ideal opportunity to introduce the topic of entropy. It has units of Joules per Kelvin for a given quantity of substance and could be added to our internal energy diagram.
When heat is given to the gas in the Carnot engine, then the entropy rises (isothermal heat flow on the hot reservoir) and we see constant entropy for both adiabatic curves as there is no heat flow in these regions. Finally, the entropy falls when the gas gives out heat to the cold reservoir. Although a greater quantity of heat is given to the gas from the hot reservoir then is discharged by the gas to the cold reservoir , when the ratio "heat to temperature" is taken then we see that both ratios are numerically equal.
Now, although the public at large has given this quantity an almost sinister characteristic "entropy death " and the like, we are happy to accept entropy as a measurable parameter in science.
The following example shows how entropy is needed to specify physical changes.
Assume that the cylinders have equal mass, m. and equal specific heats, s then it is easy to show that the internal energy ( before to after) is not changed when the cylinders are joined together --- Hot cylinder gives heat energy, Q, (= m*s*(TH - TC)/2 ) to the cold cylinder but the internal energy of the "combined" cylinder does not change. If we now calculate an entropy change we see that the hot cylinder has a negative change -Q/TH and the cold cylinder has a positive change Q/TC and so the total entropy change is Q*(TH - TC)/(TH*TC ) . At once we see that this experiment is not reversible as the entropy change is positive and yet all the other parameters have given us no indication of this.
Thus, for any tranformation/ interaction we have to monitor ENTROPY and if there is no change then it is a reversible transformation whereas a change signifies irreversibility. Why entropy is introduced at this stage of Thermodynamics is that Carnot deals with a reversible cycle and the quotient Q/T is easily visualized from the Carnot cycle.
Just an aside about text books----- It is unlikely that Hoare's book will be available now and a second best is the text by E. M. Goodger " Principles of Engineering Thermodynamics". The second edition was printed in 1984 and is still widely used. A brief treatment of the Carnot Cycle is given on page 39 and is included below:=

And now we make a summary of the final topic; entropy
Entropy
It is rather like a Murphy Law applied to Physics -- "if an unhappy event CAN occur then it WILL occur" eg if a buttered piece of toast falls on the floor it will fall butter side down ! A more disordered world exists after any interaction and, whereas Physics Laws can usually be played forwards or backwards, this is one area where there is a well defined arrow -- a glass of water, when spilt, will never re-assemble to become a glass of water again.
Two entries in Wikipedia are given
Arrow of time
Main article: Entropy (arrow of time)
Entropy is the only quantity in the physical sciences that seems to imply a particular direction of progress, sometimes called an arrow of time. As time progresses, the second law of thermodynamics states that the entropy of an isolated system never decreases. Hence, from this perspective, entropy measurement is thought of as a kind of clock.
Cosmology
Main article: Heat death of the universe
Since a finite universe is an isolated system, the Second Law of Thermodynamics states that its total entropy is constantly increasing. It has been speculated, since the 19th century, that the universe is fated to a heat death in which all the energy ends up as a homogeneous distribution of thermal energy, so that no more work can be extracted from any source.
As you see -- entropy is a fastinating subject.