Session 3 Charge and ELECTROSTATICS
As reported by the ancient Greek philosopher Thales of Miletus around 600 BC, electrical charge could be accumulated by rubbing fur on various substances, such as amber. The Greeks noted that the charged amber buttons could attract light objects such as hair. They also noted that if they rubbed the amber for long enough, they could even get an electric spark to jump. Thus, the subject of electrostatics was born but it was many years later before the science of electricity was understood.
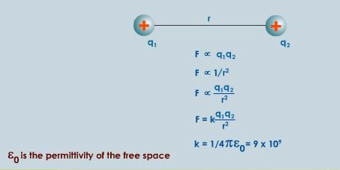
Coulomb (1736-1806), a French Physicist, put the subject onto a quantitative basis when he announced his law concerning the force between charges:

It is quite instructive to watch a UTube presentation showing the torsion balance that was used and see the similarities to the Cavendish (1731-1810) experiment conducted at about the same time.
An inverse square law given by Coulomb is very similar to Newton's gravitational law but, of course, there are repulsive forces in electrostatics as well as attractive forces. Also the magnitude of the electrostatic force is much larger then the gravitational force.
The story of charge is well presented by Lewis Ryder (Charge, Physics Education 42(2), 141, March 2007) and we see how electrical potential and electric fields are introduced.
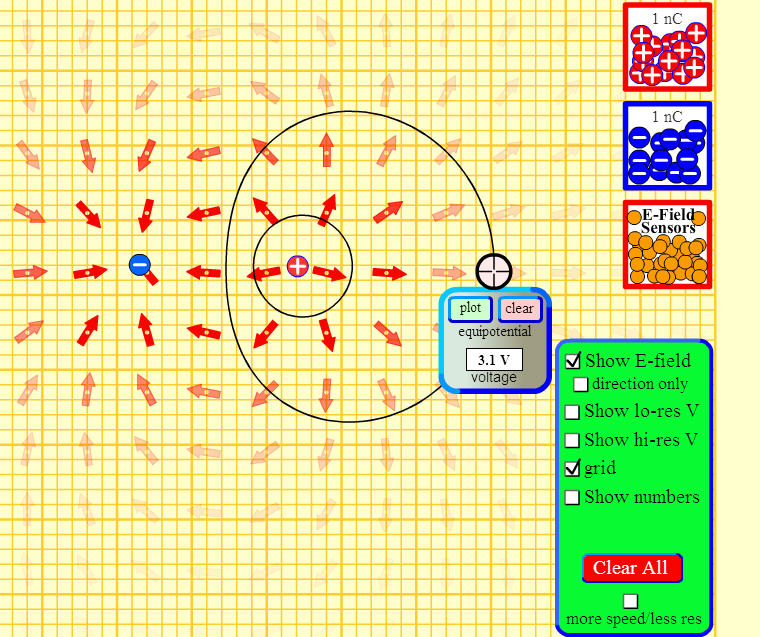
Many simulation packages are available to reinforce the concepts of fields and potential. A very user-friendly piece of software has been produced by Colorado University. If one inspects Lab 2 on the link below and proceeds to page 10, the software can be opened. If the RUN botton is pressed then charges can be loaded onto a grid and fields and potentials can be studied.
The grid pattern where a positive and negative charge has been placed is below:-
With little effort it is possible it is possible to create simulations for electrodes ( say, a parallel capacitor) and see how fields and potential patterns indicate fringing effects.
A more advanced piece of software comes from Quickfield. A student's version is available free of charge but the mesh size is limited to 255 points and this may be a limitation. An example for conductors intersecting at right angles is given below
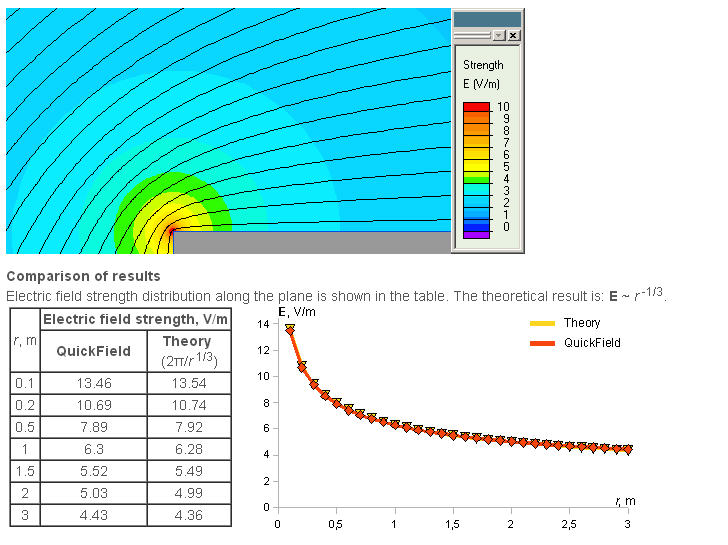
********************************************************************************************************
There is no doubt that electrostatics ( where charges are fixed ) has had less of an impact on society than current electricity. The latter has given us computers, the supply grid for powering our homes and a host of domestic and comercial equipment that define our age. However, in the formative years of electricity one of the measurement , namely, the determination of the charge on the electron by Millikan stand out as noteworthy. The video below describes how the experiment is carried out:-
Leybold -Didactic have designed and apparatus that allows students to carry out this measurement and the currently accepted value is ---------- e = 1.6021927 * 10-19 C
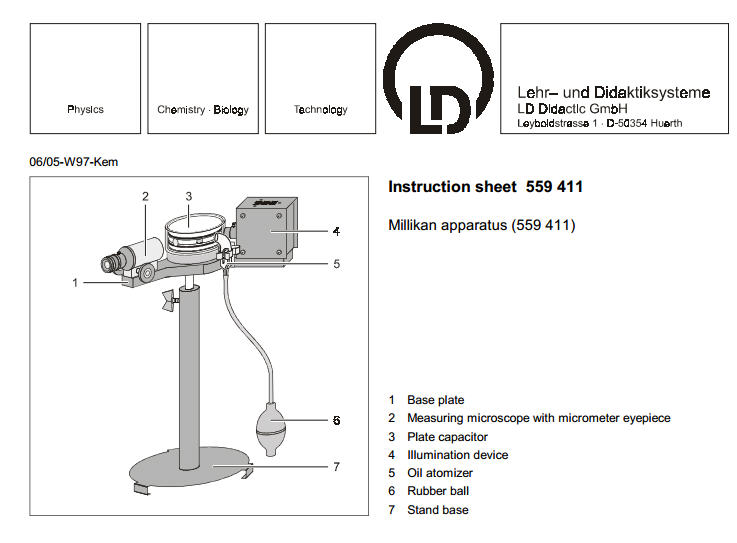
Experimentation
One can start investigating charge with very primitive equipment - after all, charging by friction (fabrics rubbed against amber) was how the Greeks were led into the subject. The "so-called" triboelectric series is as follows:-
Triboelectric Series
Air, skin (human hands), rabbit fur, glass, human hair, mica, nylon, wool, lead, silk, aluminium, paper, cotton, steel, wood, Lucite (Perspex), amber, soft rubber, hard rubber, mylar, nickel, copper, silver, brass, synthetic rubber, gold. platinum, sulfur, acetate, rayon, polyester, celluloid, polystyrene, acrylic, cellophane, polyurethane, polyethylene, polypropylene, vinyl (PVC), Teflon (PTFE) silicone rubber and ebonite. Materials from the front of the list have a tendency to give up electrons whereas those towards the end if the list have a tendency to take electrons.
An example
When a balloon is rubbed against one's hair, charge is transferred. The balloon material and human hair are such that when they are rubbed together, charge moves from one to the other. According to the above series rubber will take electrons from one's hair. So the balloon is now a charged object with a negative charge. When the balloon is brought near a wall, the wall is polarized. The wall's negative charges move away from the balloon and the wall's positive charges remain in place. So although the net charge on the wall is still zero, the wall will behave as if it is positively charged in the vicinity of the balloon. Since the balloon is negatively charged and the wall near it is positively charged, the balloon is attracted to the wall. The force of attraction is sufficiently strong that the balloon 'sticks' to the wall and defies gravity.
Experiments concerning potential and fields are described in the Leybold leaflets P3.1.3.3 "Potential inside a parallel plate capacitor " and P3.1.3.4 which examines "the potential around a charged sphere". The Physics Education journal articles:-: "Applications and teaching of bioelectrostatics" vol 42 (2) page 133, 2007 and " An Electronic Electroscope" vol 49(1) page 18, 2014.
**********************
Details of the electronic electroscope are given in the article below:
An Electronic Electroscope - a short note
Introduction
The subject of electrostatics continues to fascinate students and recently Ganci [1] has
demonstrated some interesting experiments relating to charged materials and has
imaginatively used an empty food can with a strip of kitchen foil to produce a creditable
leaf electroscope.
In this short note a description will be given of how to modernize an electronic
electroscope designed by Davies [2] several decades ago. Although the cost of the present
instrument will undoubtedly be higher than that of the leaf electroscope presented by
Ganci [1] an electronic output adds greatly to the versatility of any electroscope. It is
hoped that such an instrument will further enhance the teaching of electrostatics.
Charge Amplifier
The circuit has few components and is shown in Figure 1
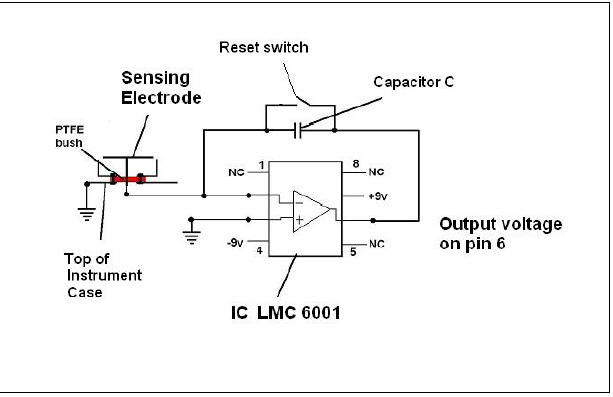
Figure 1 Charge measuring circuit ( NC signifies a pin that is not connected )
The electrode assembly follows from [2] and the low leakage IC is a National
Semiconductor device, LMC 6001. A similar circuit has recently been presented by
Dinca [3] where an IC LMC6081 has been used but both the 6001 and 6081 have
acceptable specifications. The silver mica capacitor, C, has value 1.8 nF .
From [2] and [3] it is seen that charge, Q, on the electrode produces a voltage output, V,
where :
V = Q/C
Hence, with C being a constant, the charge, Q, can be calculated from the measured
voltage.
Constructional details are given in Figure 2 (a) (b).
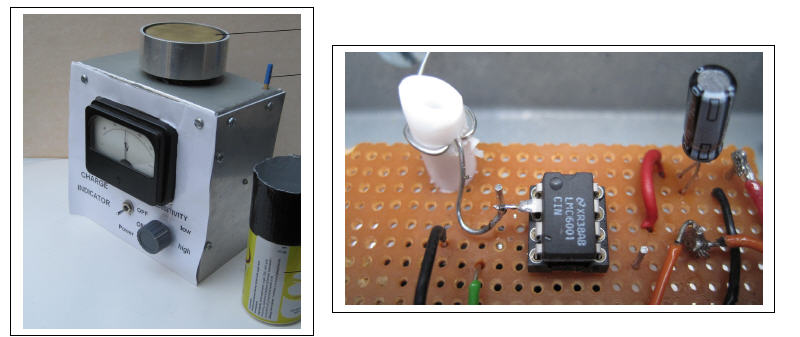
(a) (b)
Figure 2 Practical details for the electronic Electroscope
A centre zero meter indicates either positive or negative charge deposited/ placed on the
top sensing electrode, Fig 2 (a), and two resistors are switched in series with the meter to
give low/ high sensitivities. The reset switch is a rod protruding from the top of the
instrument case. It is a direct copy of that given in reference [2] and shorts the capacitor
when pushed down. Power is provided by two 9 volt batteries.
A beverage can, at the right hand side of the instrument, is a Faraday cup and is used
when measurements on charged liquids or granular samples are being made.
At the heart of the electroscope is the low leakage IC, namely, LMC 6001. The data sheet
claims that an ultra low leakage of better than 10 fA is possible with this device and, page
9 of the data sheet, gives a suggested layout for a printed circuit board. However, the
author has used a much simpler layout on veroboard. By bending pin2 so that it can be
attached directly to a PTFE post screwed to the board (Fig 2 (b)) the operation of the
device is not degraded in any way and an output free from drift is achieved.
Experiments
The meter reading responds to charged objects being placed near the sensing electrode
and a Picoscope ADC-100 captures any readings from the meter. Voltage readings can
be approximately related to charge readings ( say picoC ) by connecting a charged
capacitor from sensor electrode to earth. Figure 3 shows the results of such connections
with a capacitance of 100 pF charged to 20 volts.
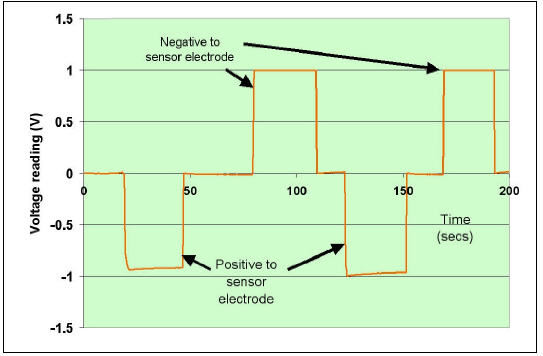
Fig 3 Graph showing calibration of the electronic electroscope
In Fig 3 it is seen that a 1 V reading correspond to about 2000 pC and that a negative
reading implies a positive charge on the electrode.
In Fig. 4 a charged PTFE is swung over the electroscope showing that it responds to
dynamic or static charges presented to the sensing plate. The lower part of the graph
shows the presence of a charged glass rod and the accumulated charge will be positive for
this material
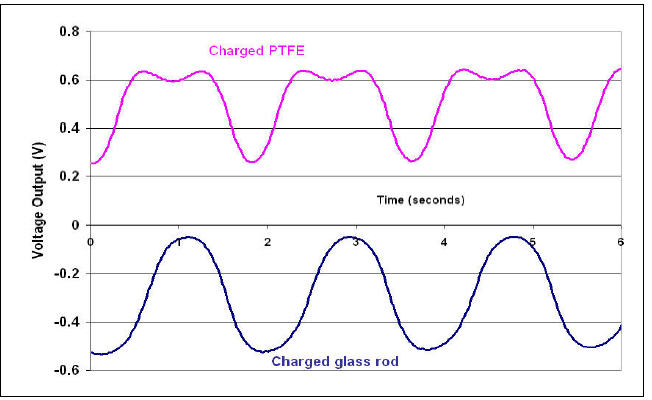
Fig 4 Charged rods swung to and fro over the electroscope
Glass rubbed on wool becomes positively charged and the readings on the electroscope
are negative whereas PTFE acquires a negative charge when rubbed over one’s garments
and a positive deflection occurs. (The triboelectric series (simplified) is given in the
Appendix)
With the Faraday cup placed on the electrode, a de-ionised water sample was poured
from a reservoir into the cup. Figure 5 shows how the voltage changes with time
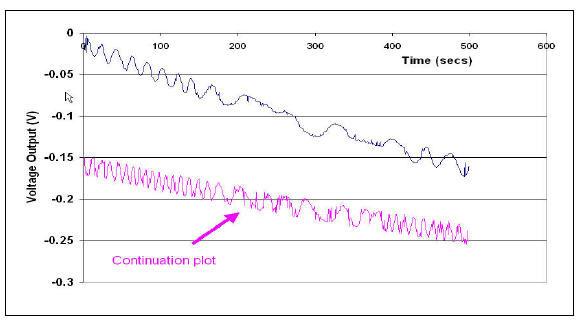
Fig 5 Negative voltage due to accumulated water drops falling into the Faraday can
The continuation plot has a smaller gradient than the initial line but this is due to a fall in
the liquid level in the reservoir. The “noise” on the curves is instrumental and could
largely be removed by placing a capacitative filter between the output of the IC .and
earth. The author must confess to having built in prejudices from times past in watching
pen movements on chart recorders. “A little noise on the trace indicates activity and
responsivity of the instrument” was the saying at the time.
Conclusion
An electronic electroscope has been constructed based on the ultra low leakage device
LMC 6001. The instrument design follows very closely that given by Davies [2] but is
up-dated with a superior IC and the provision of data logging. The results show that the
electronic electroscope offers quantitative data as compared to the leaf electroscope and it
is felt that this instrument could readily be constructed in the laboratories of schools and
colleges and would add to the teaching of electrostatics.
References
[1] S. Ganci (2012) “A portable electrophorus device” Phys. Ed., 47(1), 12-14.
[2] G. R. Davies (1974) “An electronic gold leaf electroscope” Phys. Ed., 9(6), 393 –
398.
[3] M. P. Dinca (2011) “Charge sniffer for electrostatic demonstrations” Am. J. Phys.,
79(2), 217-221.
A very informative article on the importance of electrostatics in the industrial world
has recently been given by Ian Pavey, Chilworth Technology Ltd.
Appendix
For a good introduction to the subject of electrostatics it is suggested that one watches a
video from MIT OPEN COURSEWARE, Electrostatics, “Highlights for High Schools”
Triboelectric Series
Air, skin (human hands), rabbit fur, glass, human hair, mica, nylon, wool, lead, silk,
aluminium, paper, cotton, steel, wood, Lucite (Perspex), amber, soft rubber, hard rubber,
mylar, nickel, copper, silver, brass, synthetic rubber, gold. platinum, sulfur, acetate,
rayon, polyester, celluloid, polystyrene, acrylic, cellophane, polyurethane, polyethylene,
polypropylene, vinyl (PVC), Teflon (PTFE) silicone rubber and ebonite.
Materials from the front of the list have a tendency to give up electrons and hence
become positive whereas those towards the end if the list have a tendency to take
electrons and thus become negative.
An example
When a balloon is rubbed against one’s hair, charge is transferred. The balloon
material and human hair are such that when they are rubbed together, charge
moves from one to the other. According to the above series rubber will take
electrons from one’s hair. So the balloon is now a charged object with a
negative charge.
When the balloon is brought near a wall, the wall is polarized. The wall's
negative charges move away from the balloon and the wall's positive charges
remain in place. So although the net charge on the wall is still zero, the wall
will behave as if it is positively charged in the vicinity of the balloon. Since the
balloon is negatively charged and the wall is acting as if it is positively
charged, the balloon is attracted to the wall. The force of attraction is
sufficiently strong that the balloon 'sticks' to the wall.
********************************************
Quadrant Electrometer
This is an instrument that goes a long way back in history. Over 100 years ago it was the prefered instrument for measuring electrical voltage and power.

The theory is quite simple and is outlined in the attached file Theory_Q_E.pdf
The construction of the Quadrant Electrometer has been described many times and the article by Sutton is probably more complete than most Sutton_QE.pdf
The construction of a simplified version of the instrument can be carried out as a student project and the article by L W Brown in the Physics Education journal is a good starting point (1985 , volume 20, p287). The photograph show a working model made by the author.
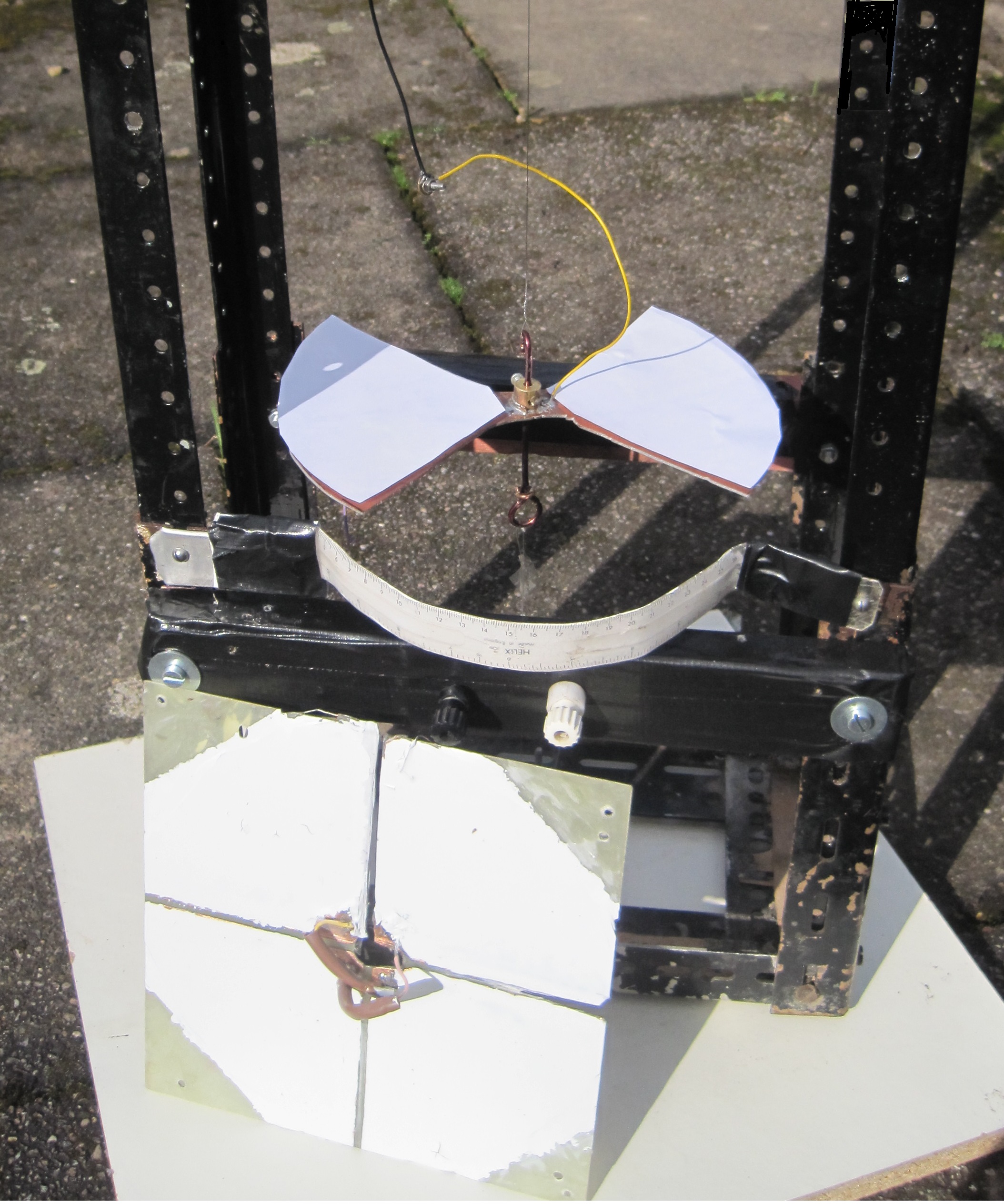
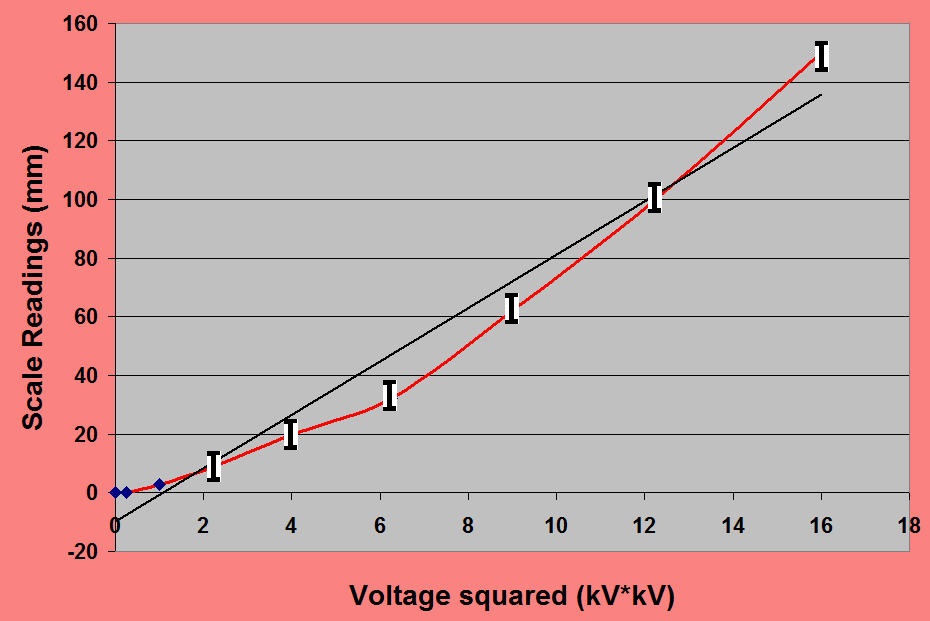
The vane electrode is suspended above the quadrants and the frame is approximately 1 m high, Readings in the range 0 to 4 kV were recorded as shown below:
The constructional details are based on those given by Brown.
Ion Power
The quadrant electrometer constructed by Brown was used to measure details of Atmospheric Electricity and recently there has been commercial interest in "harvesting" this power to provide a sustainable source of energy https://ionpowergroup.com . More details are given in the attached file Ion_power.pdf .
One hopes that such energy installations will provide a significant output for our planet earth or, for instance, Mars when settlements are established there.
CORONA DISCHARGE
Most people know about arc /spark discharge through spectacular lightning in the sky or the more mundane discharges in gas lighters or the sparks in engine spark plugs. However there is also a quieter form of electrical discharge called corona discharge and this has been used for many decades in such things as fly-ash precipitators, paint spayers and Xerox copying machines. More recently it is being used for household items such as air purifiers and ionic hair dryers. An example of a plug-in purifier is shown below:-

A Scientific American article Sci_Am_Adam1.pdf and some work that was carried out at Manchester Polytechnic, Scott_Adam2.pdf should help to give a fuller explanation of how corona discharge works.